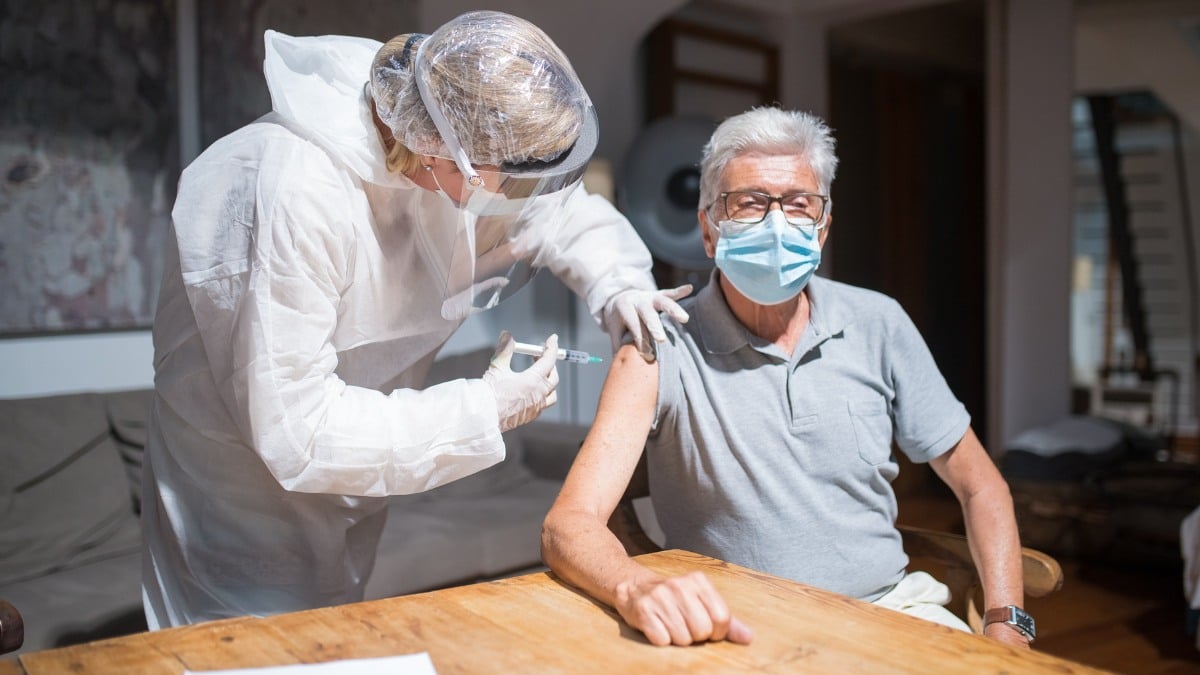
The US Food and Drug Administration – FDA authorizes 2nd dose of Covid Booster for older people and individuals with weakened immune systems.
People aged 65 and above can get the second dose of the updated versions of Pfizer-BioNTech and Moderna’s Covid-19 booster at least four months after their last dose, while most people who are immunocompromised can get an additional dose at least two months after their last one.
The FDA also authorized the use of the bivalent formula in all Covid-19 vaccines moving forward, not just for booster shots.
The CDC has scheduled a meeting with its panel of outside advisers on Wednesday to vote on the FDA’s decision. Once approved by the CDC, people can get the updated vaccines immediately. The booster shots were reformulated in August 2022 to target the BA.4 and BA.5 omicron subvariants, in addition to the original strain of the virus.
According to a CDC report, the updated Covid-19 boosters reduced the risk of Covid-19 infection from the XBB.1.5 subvariant by almost 50%, and another study conducted by Israeli researchers showed that the boosters reduced the risk of hospitalization in people 65 and older by 72%.
Even though the boosters do not match the currently circulating strain, infectious disease specialist Dr. Isaac Bogoch said that they should still provide people with some protection.
Despite the FDA authorization, uptake of the boosters has been low, with only approximately 17% of the total US population having received one, according to the CDC. The FDA’s decision follows several months of doubt surrounding the move to yearly Covid-19 boosters for most adults and children.
However, the FDA appeared supportive of multiple doses for the most at-risk Americans, such as the elderly or people with weakened immune systems.